Discovering existing drugs that can be used against COVID-19
MAKE A GIFT TODAY TO HELP US FIND SUCCESSFUL TREATMENT OPTIONS AS WE CONTINUE TO FIGHT CORONAVIRUS
The possibility that SARS CoV-2 vaccination or infection may not produce long-lasting protection, and the existence of immunocompromised or other individuals who don’t mount long-lasting immune protection, emphasize that there is a great need for identification and development of more effective antiviral agents that act directly against the SARS CoV-2 virus.
With our collaborator, Dr. Ram Samudrala in the UB Department of Biomedical Informatics, we (in the UB Department of Microbiology & Immunology) used his NIH-funded state-of-the-art computational program to predict which of the ~3,000 current FDA-approved drugs are likely to target critical SARS CoV-2 viral enzymes for potential anti-viral treatments; ~200 drugs were identified predicted to target important viral enzymes.
Our group is currently assembling, validating, and deploying two novel cell-based assays we have designed to evaluate functions of vital SARS CoV-2 enzymes. These assays will be used to test the ability of the identified FDA drugs to effectively inhibit these viral enzymes inside human cells in the absence of an ongoing viral infection (to essentially ‘isolate’ particular viral functions, but still in living human cells). Those drugs that show high function and specificity against their predicted viral enzyme target will then be tested for inhibition of SARS CoV-2 infection of human lung cells (by our UB colleague Dr. Stavrou).
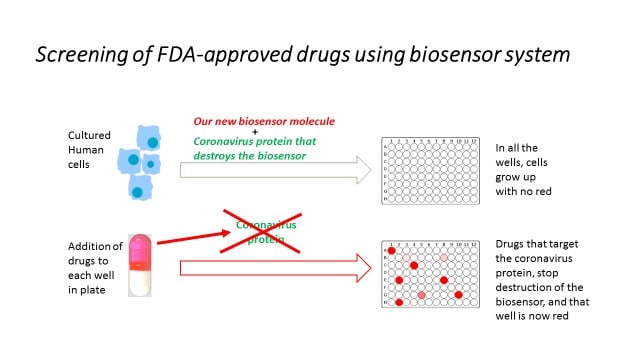
Until now most drugs for use against COVID-19 act indirectly by affecting the host immune system. Repurposing existing drugs for use against SARS CoV-2 would be a real game-changer, as FDA-approved drugs have already been tested for safety in humans and are already present in pharmacies.
They could be prescribed immediately following a positive COVID-19 test, protecting that individual against progression to serious disease, as well as suppressing virus production and spreading to other individuals. (The first approved human drug predicted to target one of these viral enzymes, masitinib, was just reported in September 2020, using a new testing approach similar to ours. This drug appears in the approved drug panel we published; however, our list includes other FDA-approved drugs that are predicted to have even higher efficacy against COVID-19. Better drugs against SARS CoV-2 are out there – and our approach can find them!)
Please help us fight this pandemic. Tax Deductible donations to this research can be made here!
Note all contributions to the Papillomavirus Fund will be directed to COVID-19 drug development for the duration of the pandemic.


